DISSOLVE BIOFILMS

ONE LESS RISK IN INFECTION CONTROL

enziQure
®
Highly dosed enzymatic cocktail for corrective cleaning of persistent microbial Biofilms contamination on Medical Devices
ONELIFE ENZYMES REDUCE RESISTANCE & PERSISTENCE
-
Resistance : preventing exchange of antibiotic-resistant
genes by dissolving biofilms and reducing systematic use
of disinfectants, reduces antimicrobial resistance.
-
Persistence : A key factor in pathogen transmission is reduced
by exposing pathogens before high-level disinfection.
-
OneLife's enzymatic technology enables complete and irreversible
degradation of organic soil and biofilm, they enable complete
disintegration of organic soil and biofilm matrix for completely clean
instruments and optimal risk management.
INDEPENDENT LABORATORY TESTS
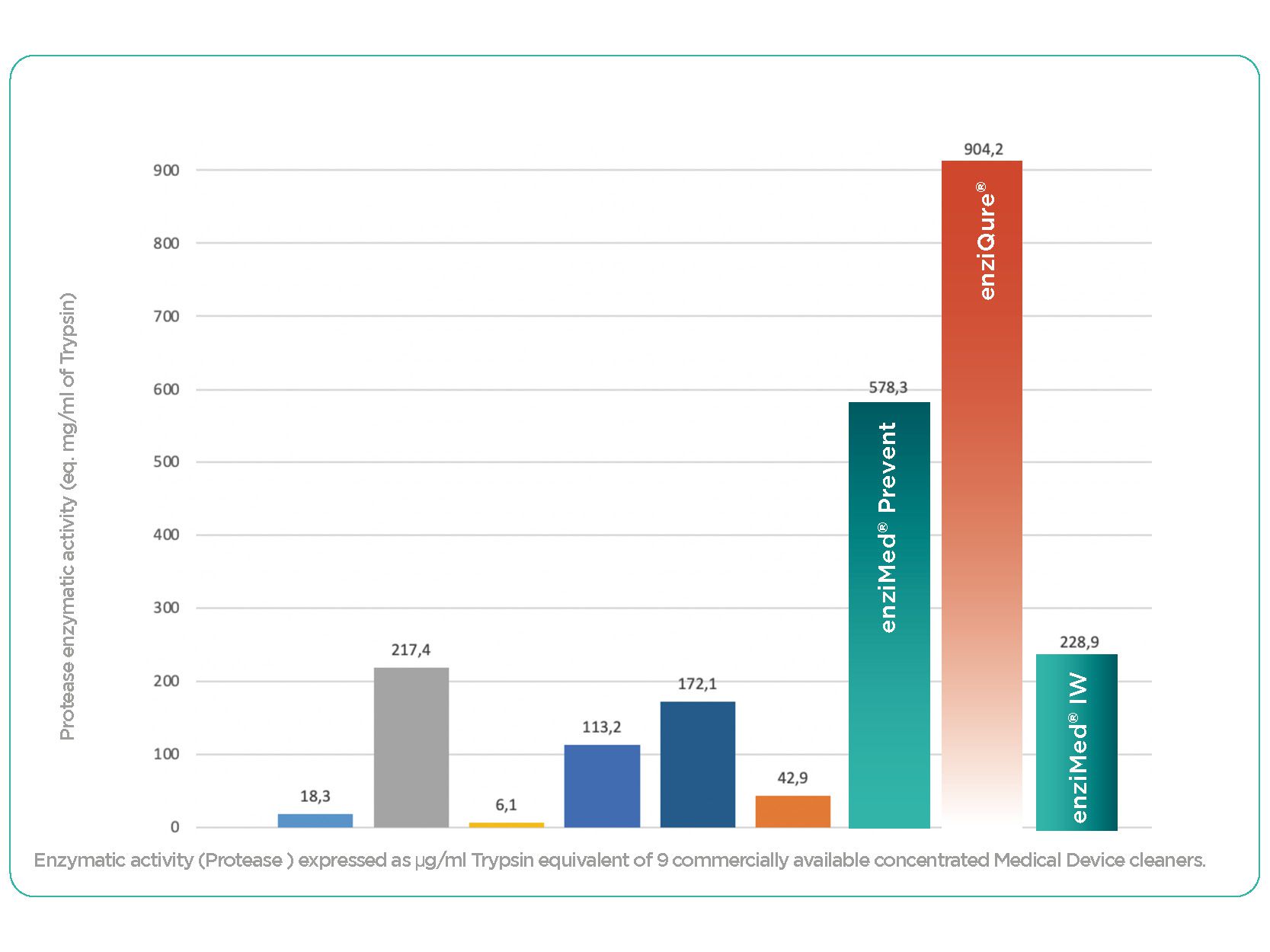
Conclusion of the independent Laboratory Meurice tests: “OneLife detergents outmatch the competing products by factors ranging from 4.15 to 86.5 in terms of protease activity levels. Being charged with high levels of protease activity, OneLife products ensure that the cleaning of proteinaceous matter from Medical Device is fast and effective.” More details on demand
REDUCES CONTAMINATION OF ALL TYPES OF BACTERIAL STRAINS
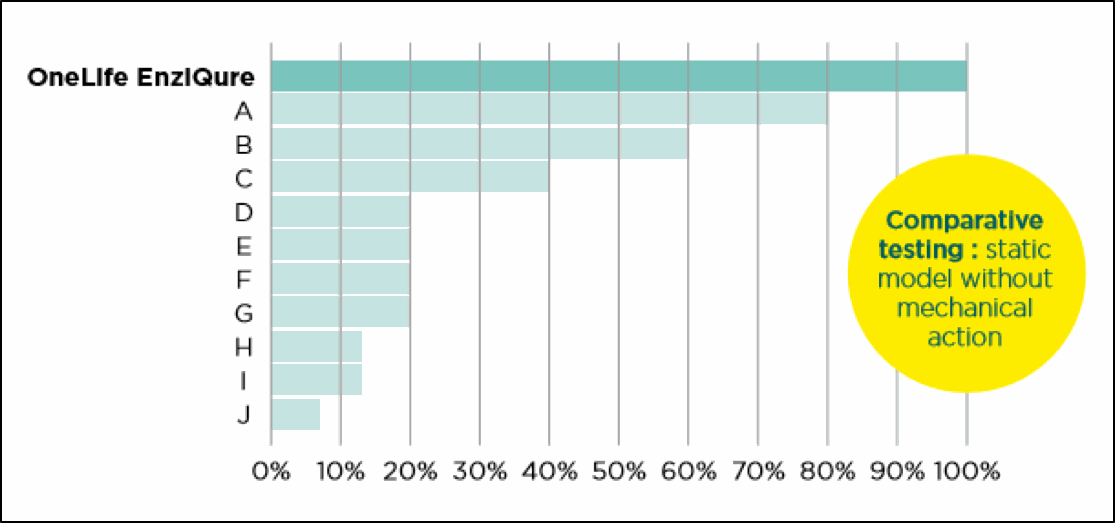
COMPARATIVE TEST ON DISINTEGRATION OF BIOFILM MATRIX
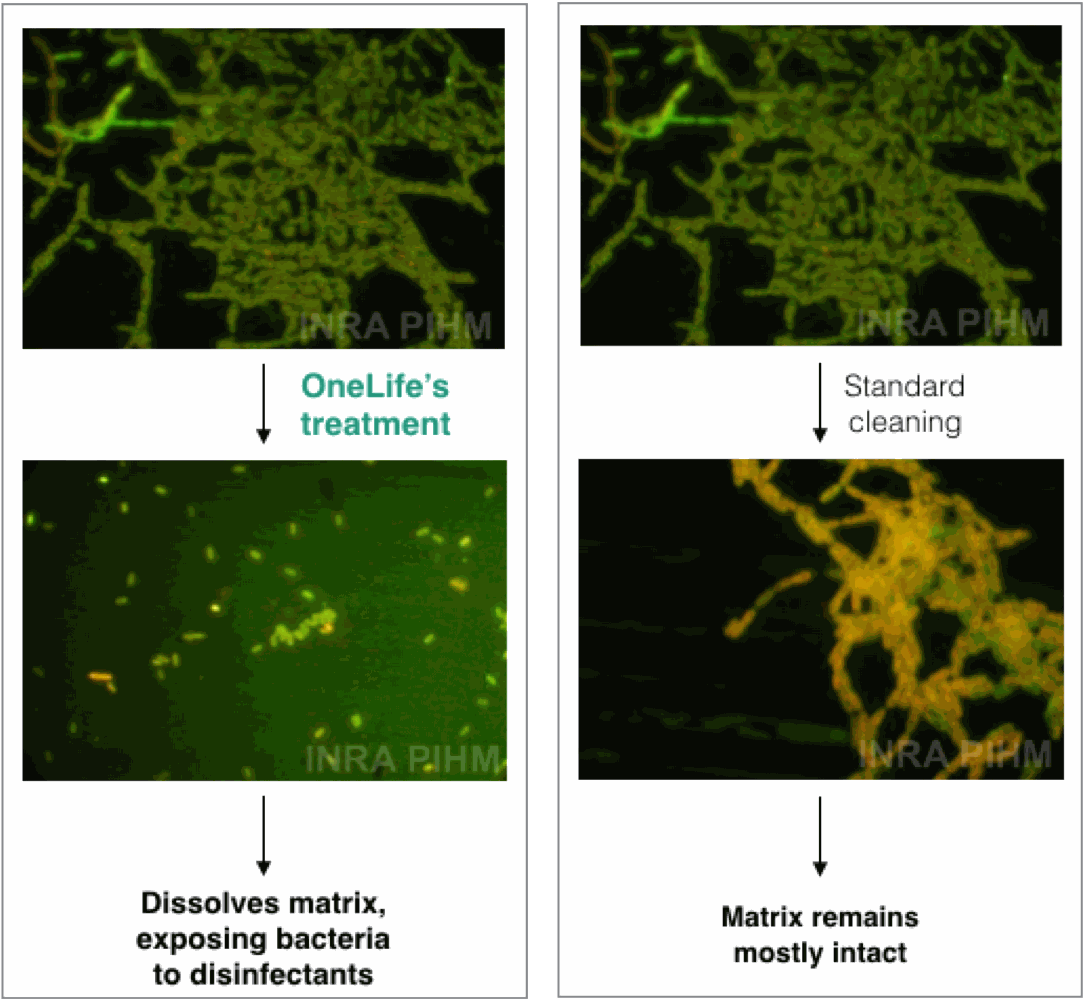
CONDUCTED BY INRA *National Institute of Agronomical Research, France
Due to the complexity and heterogeneity of extracellular polymeric substances (EPS) from different biofilms, a blend of enzymes is required for degradation of biofilm matrix. OneLife's multi-enzymatic compounds
are the only detergents to disintegrate biofilm matrix of 100% of tested strains. Other detergents attack 0 to 53% of strains.
Louvain Drug Research Institute of the Université Catholique de Louvain. Ability of 11 different Medical Device detergents to remove over 35% of biomass on 15 different strains of biofilm.
Note: tests are conducted without mechanical action in order to achieve a direct comparison of detergent quality.
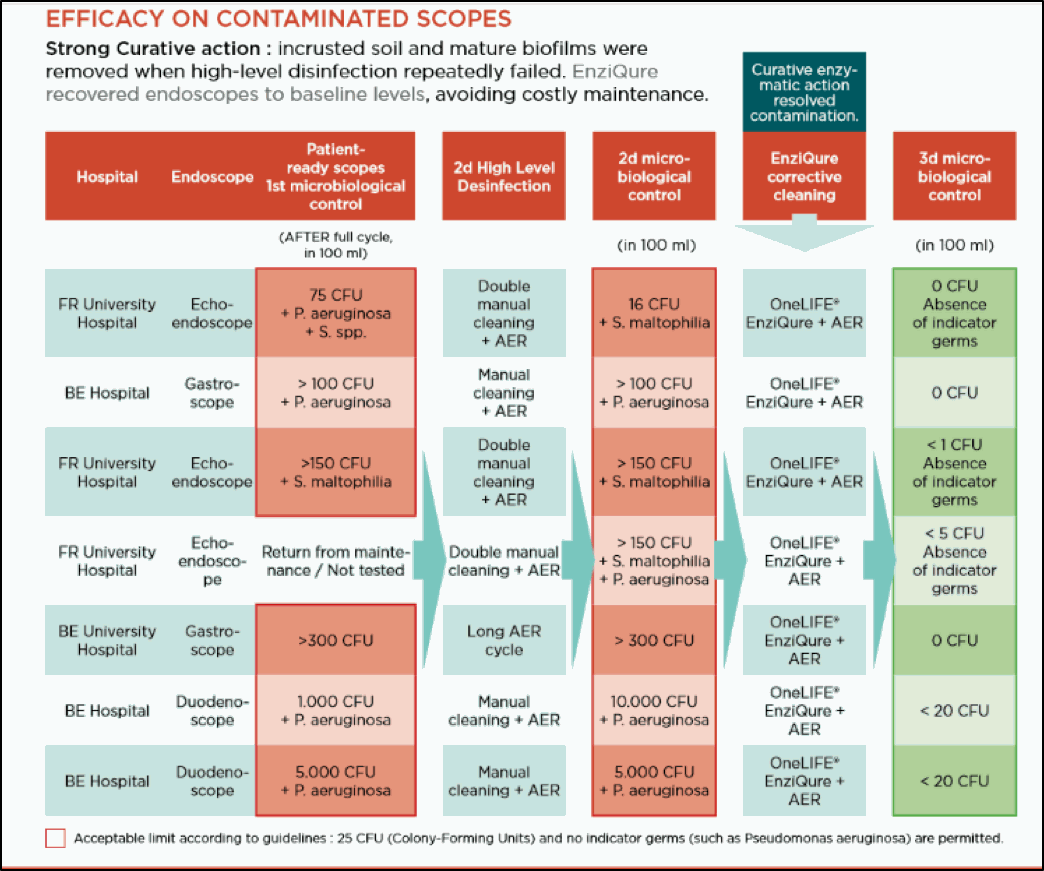
EFFICACY ON CONTAMINATED SCOPES
Incidents occur despite the thorough decontamination protocols in place in hospitals. These tests were run on multiple patient-ready endoscopes. Current standards of cleaning and disinfection have displayed limitations when confronted with biofilms. A single curative treatment with enziQure® followed by high-level disinfection enabled reduction of microbiological contamination to acceptable levels.